
Delivering Disease Prevention®

PROCESS
Different Species – Different Routes – Different Vaccines
Each animal species is different, as are its feeding habits, its environments, and its microbiome. Understanding an animal's digestive tract is essential in delivering vaccines to the right place in the animal's intestines.

Field Mouse
- Diverse genetic background
- Can carry multiple pathogens
- Outdoors
- Large numbers
- Multiple environments
- Uncontrolled eating habits
- Single-stomach digestive tract
Canine
- Mixed-breed or pure-breed
- Indoor or outdoors
- Multiple sizes / breeds
- Large numbers
- Controlled or uncontrolled eating habits
- Strong preferences for certain foods
- Single-stomach digestive tract
Poultry
- Controlled breeding
- Food animal
- Multiple stocks and lifespans (breeder, broiler, layer)
- Large numbers
- Special feed / water requirements (focus on nutrition)
- Single stomach with crop digestive tract
Livestock
- Controlled breeding
- Multiple breeds / sizes
- Food animal (meat and milk)
- Long lifespan
- Special feed / water requirements (focus on nutrition)
- Multi-stomached digestive tract
Human
- We translate what we know about digestive systems to develop human vaccines as well, including a novel orally delivered flu vaccine that can be delivered to your home.
COMPONENTS


Substrate Component Features
- Integration of species-specific substrates that allows integration of multiple vaccines, therapeutics, and additives, each of which may be incorporated as an amalgam or as a spray-on.
- Targeted gut placement that maintains in harsh GI environments.
- Coarse textures, providing mechanical stimulation of the animal’s innate immune system.
- Kibble, chewables; effervescent tablets, and powders.


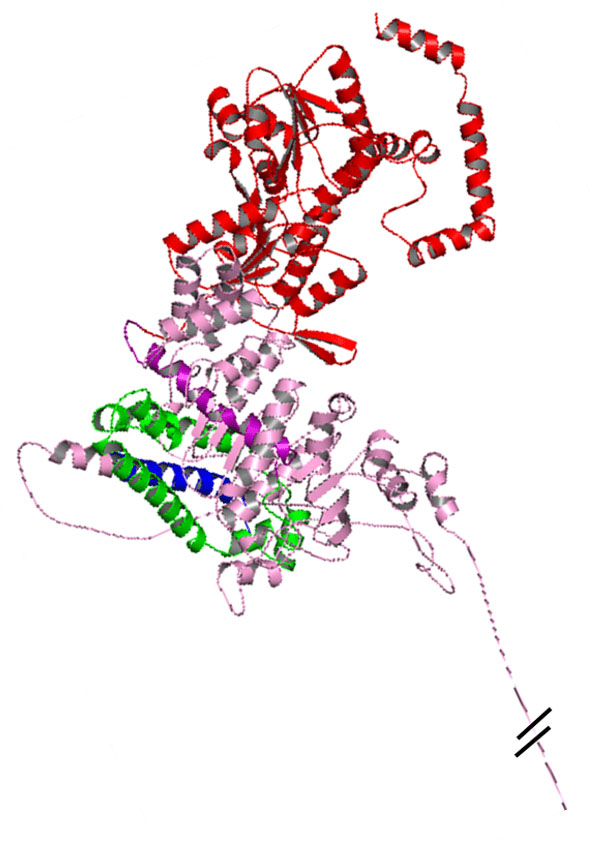
Bacterial Expression Vehicle Component
-
Vehicle inactivation (non-GMO, thermostability).
-
Recombinant antigen expression system (AVOIDS use of infectious agents).
-
Molecular adjuvantization (cost reduction / no chemical complications).
- Microbiome friendly
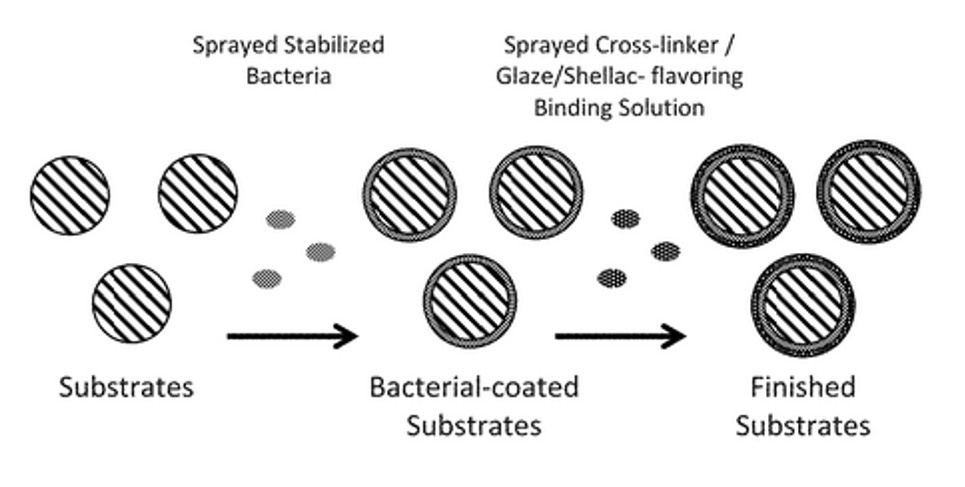
Chemical Encapsulation Component
- Targeted gut placement.
- High-throughput, low-cost, and resistant to heat.
- Thermostability
MANUFACTURING
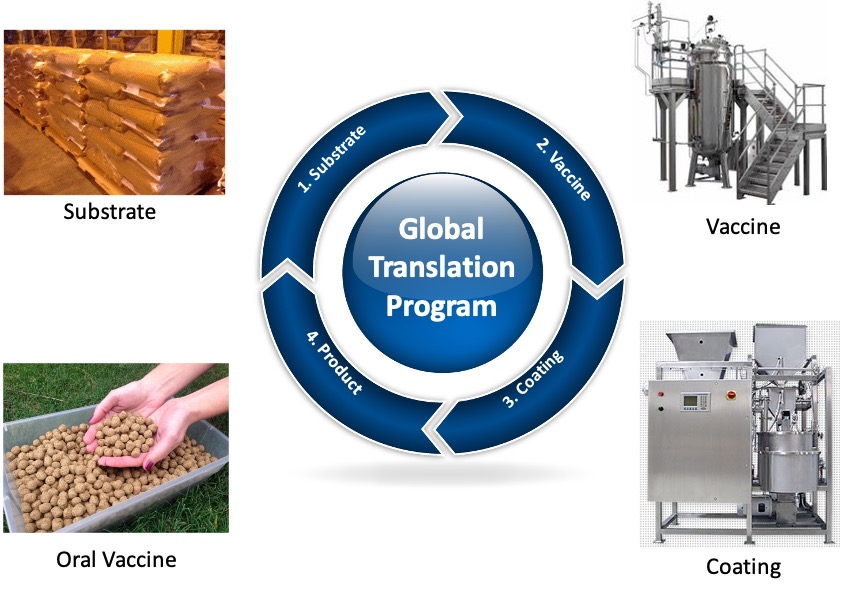
Manufacturing Features
-
No lyophilization.
-
Low-cost capital equipment build / compact design.
-
Non-centralized manufacturing.
-
Modular equipment design.
-
High throughput with maintained batch consistency.
PUBLICATIONS
- Wickramasuriya SS, Park I, Lee Y, et al. Effect of orally administered B. subtilis-cNK-2 on growth performance, immunity, gut health, and gut microbiome in chickens infected with Eimeria acervulina and its potential as an alternative to antibiotics. Poult Sci. 2024 Nov;103(11):104156.
- Wickramasuriya SS, Park I, Lee Y, et al. Orally delivered Bacillus subtilis expressing chicken NK-2 peptide stabilizes gut microbiota and enhances intestinal health and local immunity in coccidiosis-infected broiler chickens. Poult Sci. 2023 May;102(5):102590.
- van Oosterwijk JG, Wikel SK. Resistance to Ticks and the Path to Anti-Tick and Transmission Blocking Vaccines. Vaccines (Basel). 2021;9(7):725.
- Wickramasuriya SS, Park I, Lee Y, et al. Oral Delivery of Bacillus subtilis Expressing Chicken NK-2 Peptide Protects Against Eimeria acervulina Infection in Broiler Chickens. Front Vet Sci. 2021;8:684818.
- van Oosterwijk JG. Anti-tick and pathogen transmission blocking vaccines. Parasite Immunol. 2021 Mar 11:e12831.
- Williams SC, van Oosterwijk JG, Linske MA, et al. Administration of an Orally Delivered Substrate Targeting a Mammalian Zoonotic Pathogen Reservoir Population: Novel Application and Biomarker Analysis. Vector Borne Zoonotic Dis. 2020 Mar 26.
- Stafford KC 3rd, Williams SC, van Oosterwijk JG, et al. Field evaluation of a novel oral reservoir-targeted vaccine against Borrelia burgdorferi utilizing an inactivated whole-cell bacterial antigen expression vehicle. Exp Appl Acarol. 2020 Feb;80(2):257-268.
- Gomes-Solecki M, Arnaboldi PM, Backenson PB, et al. Protective Immunity and New Vaccines for Lyme Disease. Clin Infect Dis. 2019 Oct 17. pii: ciz872.
- Zatechka S. Reservoir-Targeted Vaccines as a One Health Path to Prevent Zoonotic Disease. Int J Vaccines and Vaccination. 2016;6(2).
- Gomes-Solecki M. Blocking pathogen transmission at the source: reservoir targeted OspA-based vaccines against Borrelia burgdorferi. Front Cell Infect Microbiol. 2014;4:136.
- Meirelles Richer L, Brisson D, Melo R, et al. Reservoir Targeted Vaccine Against Borrelia burgdorferi: A New Strategy to Prevent Lyme Disease Transmission. J Infect Dis. 2014 Jun 15;209(12):1972-80.
- Meirelles Richer L, Aroso M, et al. Reservoir targeted vaccine for Lyme borreliosis induces a yearlong, neutralizing antibody response to OspA in white-footed mice. Clin Vaccine Immunol. 2011 Nov;18(11):1809-16.
- Gomes-Solecki MJ, Brisson DR, Dattwyler RJ. Oral vaccine that breaks the transmission cycle of the Lyme disease spirochete can be delivered via bait. Vaccine. 2006 May 15;24(20):4440-9.
INTELLECTUAL PROPERTY
- Composition and Method of an Orally Administered Antimicrobial Peptide Vectored in a Bacterial Expression Vehicle
- Composition & Method for Reducing Zoonotic Infectious Disease
- Method to Reduce Tick Population with a Universal Tick Antigen
- Method for Reducing Zoonotic Infectious Disease
- Oral Vaccine for Borrelia
- Oral vaccine for peste-des-petits-ruminants virus
- Time Release Application and Monitoring System
- Time release application and monitoring system configured to prevent untargeted wildlife entry or disruption
- Visco-elastic solid formulation for oral delivery of a biologically active agent